Large Majority of Americans Say Consideration of Clinical Trial Participation Should be a Part of Regular Health Care
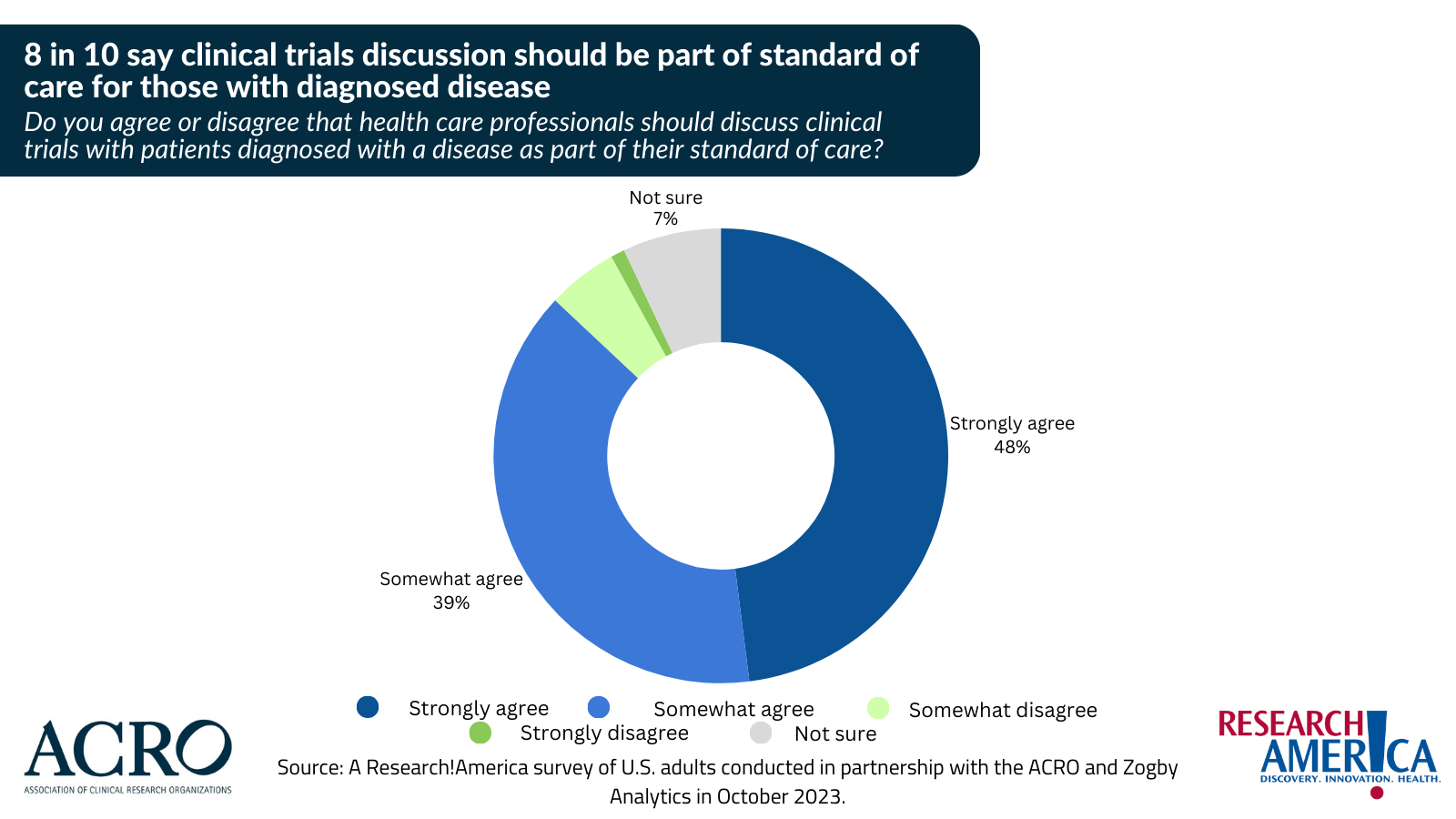
Arlington, VA – December 5, 2023 – According to survey findings released today, an overwhelming majority of Americans (87%) agree that health care professionals should discuss clinical trials with patients diagnosed with a disease as part of their standard of care. Further, sixty-one (61%) percent agree that consideration of clinical trial participation should be a part of regular health care whether patients are healthy or ill, up from 44% in 2017. Commissioned by Research!America in partnership with the Association of Clinical Research Organizations (ACRO), the October 2023 national survey captures Americans views on clinical trials and clinical research. (View slide deck of survey results.)
“We are pleased to partner again with Research!America to survey public attitudes toward clinical trials, which are essential to the development of new drugs and new treatments for the people who need them,” said Doug Peddicord, Executive Director of ACRO. “Especially important is that the public believes that clinical trials should be part of everyday health care and that physicians and other healthcare providers should discuss clinical trial options with their patients.”
Despite widespread support for the incorporation of clinical research into health care, only 36% of respondents say their doctor or other health care professional has talked with them about medical, health, or clinical research. That said, this is an encouraging jump from 19% in 2017 who said so. Overwhelmingly, respondents prefer getting information about clinical trials from their doctor or health care provider (77%) rather than other sources and say that doctors and health care providers (50%) have the greatest responsibility in educating the public about clinical trials.
While approximately 8 in 10 respondents say they have heard of a clinical trial, only 26% say they or someone in their family has ever participated in one, up from 18% in 2017. However, of those that had not participated in a clinical trial, 49% are willing to do so. Respondents noted lack of trust (57%) as the top reason individuals do not participate in clinical trials, which grew from 38% in 2017. Adverse side effects (52%), up from 34% in 2017, and lack of awareness/information (55%) follow closely.
Most Americans feel positively about clinical trials with many agreeing that they benefit from clinical research and its findings (73%). Factors important to clinical trial participation are understanding potential risks and benefits (72%), competence and reputation of people or the institution conducting the research (71%) and having an expert guide through the clinical trials process (60%). Eight in 10 respondents said they were likely to participate in a clinical trial at a traditional site (like a hospital or a doctor’s office), but many also indicated that they would be likely to participate at a non-traditional site with 75% likely to participate at a home site and 67% at a community site (like a community health center, clinic, CVS, Walgreens, etc.).
“Clinical trials are essential to progress in medical research. These new survey findings highlight that Americans are not only interested in learning more about clinical trials, they are willing to get involved,” said Mary Woolley, President and CEO of Research!America. “Though a growing lack of trust is cause for concern, it is heartening to see Americans’ strong support for clinical research. It’s clear that it’s time to expand discussion between patients and health care professionals, ensure access to clinical trials, and improve participant diversity.”
Though most respondents expressed willingness to share personal health information, the survey reveals a downward shift in public attitudes on this topic that calls for attention from the research community. Respondents say they are willing to share their health information:
- To advance medical and health research (71%), down from 82% in 2017.
- So researchers can better understand diseases and develop new cures (70%), down from 84% in 2017;
- So health care providers can improve patient care (68%), down from 79% in 2017;
- So public health officials can better track diseases, disabilities, and their causes (63%), down from 74% in 2017.
Data suggests that confidence in the U.S. system for reviewing the effectiveness and safety of new medicines and medical technologies has increased with 7 in 10 saying they are confident in the current system, up from 63% in 2017. Respondents are split, however, on the speed with which the U.S. Food and Drug Administration (FDA) should act.
Additional highlights from the Research!America/ACRO survey include:
- Seven in 10 respondents want to find out more about taking part in clinical trials.
- Eighty-five percent say it is important that clinical trials populations are representative of the U.S. population.
- Most all agree that clinical trials are important to advancing science (90%) and improving health (87%).
- Only a quarter of respondents are familiar with clinicaltrials.gov and clinical research organizations (CROs). Twenty-five percent of respondents have heard of clinicaltrials.gov, slightly up from 22% in 2017, and 24% have heard of clinical research organizations (CROs), no significant change from 2017.
The online survey was conducted by Zogby Analytics on behalf of Research!America in October 2023, among 1,005 adults plus 1,207 additional adults for minority oversampling. The survey has a theoretical sampling error of +/- 3.1 percentage points.
For questions about the survey, or to set up an interview with Mary Woolley, contact Taylarr Lopez, Director of Communications for Research!America, at 571-482-2719 or [email protected] with press inquiries.
###
About the Association of Clinical Research Organizations (ACRO)
Founded in 2002, ACRO represents the world’s leading clinical research and technology organizations, which provide specialized services that are integral to the development of drugs, biologics and medical devices. ACRO and its members advocate on a global basis for safe, ethical, high-quality medical research so patients can benefit from the development of new treatments and therapies. Our members are dedicated to helping their clients bring efficiency, innovation and value to the clinical research process.
About Research!America
Research!America is a non-profit medical and health research advocacy alliance which advocates for science, discovery, and innovation to achieve better health for all. Visit www.researchamerica.org.




