Explained: How a COVID-19 Serology Test Works And Obstacles to its Use
COVID-19 serology tests, also known as antibody tests, are currently receiving heightened attention and scrutiny. Why? Validated and correctly performed positive serology tests are a tool to confirm previous SARS-CoV2 (the virus causing COVID-19) infection and may indicate that a patient has built immunity to the virus. However, there are still questions we need to answer about the clinical effectiveness of serology testing to ensure that they yield accurate results. Additionally, scientists do not yet know for sure if a patient with a validated positive serology test is immune to reinfection by the virus.
What Must Be Learned Before Serology Tests Can Be Used Effectively
In the American Public Health Association and National Academies of Medicine’s fifth installment of the COVID Conversations Webinar series, Jill Taylor, PhD, Director of the Wadsworth Center of the New York State Public Health Department, affirmed serology testing can be confusing and that without clearer understanding and guidelines, we cannot rely exclusively on serology tests for returning to our previous “normal”.
In considering how to move forward with widespread serology testing, Dr. Taylor highlighted six questions in need of further examination. They are: 1) sensitivity, 2) specificity, 3) reproducibility, 4) specimen collection timeline, 5) state of disease and 6) when rapid diagnostic tests (RDTs) are appropriate. There are a number of research efforts underway to address these concerns. Teams at UCSF and UC Berkeley, with the backing of the Chan Zuckerberg BioHub, have been examining the sensitivity and specificity of over 120 serology tests that are currently available. They are concerned that without “systematic validation,” disease reporting could become an even larger problem and have posted preliminary data regarding some of the tests.
The FDA has recently announced that test manufacturers must submit their proof-of-accuracy data within 10 days of initial validation, or products must be pulled from the market. The FDA’s concerns arise in part from the lack of robust evidence about the test’s ability to detect differences between antibodies from the novel SARS-CoV2 coronavirus and the preexisting common human coronaviruses. A test which cannot distinguish between the two may produce a positive result even though the patient was never exposed to SARS-CoV2. Dr. Patricia Slev, Immunology Section Chief at ARUP Laboratories and Associate Professor at the Utah School of Medicine affirmed in a recent American Society for Microbiology Webinar that some evidence shows that 90% of adults over the age of 50 have antibodies to all four common human coronaviruses. Researchers are working to ensure that serology tests respond solely to SARS-CoV2 and the FDA has ramped up their requirements for serology testing as a result.
But how does the test work? What is the science behind it? In this blog, we answer these questions and hopefully shed light on the mechanism of serology tests.
The Objective of the Test
The COVID-19 serology test is designed to identify if a patient has antibodies in their blood against SARS-CoV2. Antibodies are Y-shaped proteins produced by a person’s immune system to fight off infection. A correctly performed positive serology test is designed to identify patients previously infected by SARS-CoV2. This is different from a positive diagnostic PCR test, which detects current infection. Additionally, the serology test should not be confused with antibody treatments that are also being extensively researched. At this time, “antibody treatment” means taking SARS-CoV2-specific antibodies isolated from the blood of a previously infected patient and administering them to a currently infected patient (a.k.a convalescent plasma treatment). Serology testing is crucial for understanding how many people have been infected by SARS-CoV2, if possessing SARS-CoV2 antibodies indicates COVID-19 immunity, and how to generate successful antibody treatments.
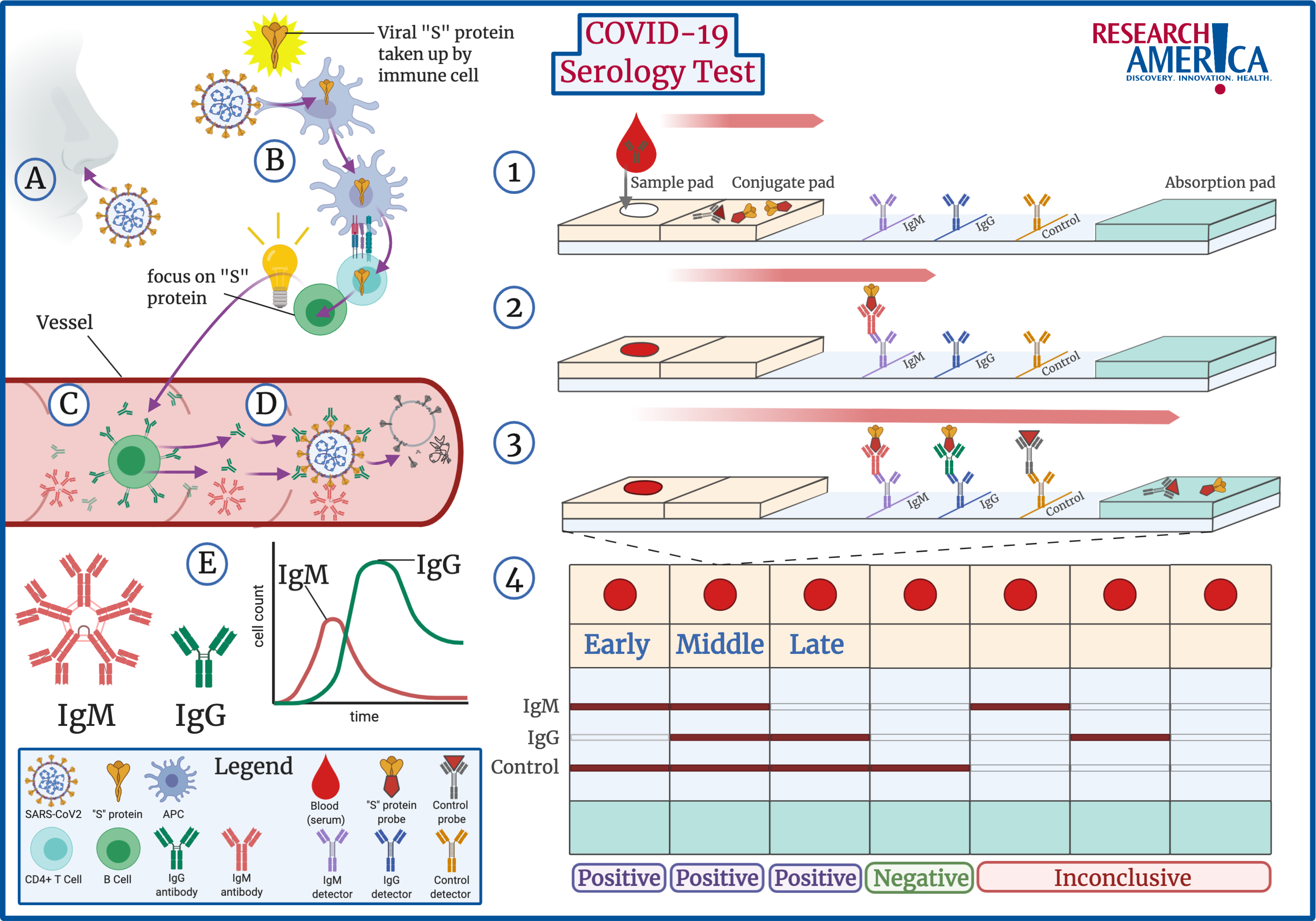
From initial SARS-CoV2 infection, to a positive serology test, the process is described below:

How Antibodies Are Developed
When a patient is infected by SARS-CoV2, the virus comes in contact with the patient’s immune system (A,B). Key immune cells identify SARS-CoV2 as a threat to the body and absorb specific information about the virus (B). In the case of SARS-CoV2, the “S” protein present on its exterior is most commonly identified, but other components can be identified as well. This information is passed to B cells, which are the key cells that produce targeted antibodies (B,C). Antibodies are the body’s best defense against infection. Anti-SARS-CoV2 antibodies are Y-shaped proteins, where the arms of the Y are specifically designed to bind to external proteins, such as the “S” protein, on SARS-CoV2. Once these antibodies are produced, they circulate in the body, attaching to every virus they see. When the virus is covered by antibodies, the body activates mechanisms to destroy the SARS-CoV2 virus (D).
Additionally, there are different types of antibodies. When a patient is initially infected, B cells produce a specific kind of antibody known as IgM antibodies. These antibodies roam in packs of five at a time and do not last long in the body (E, Left). Over time, IgM antibodies are replaced by IgG antibodies, which roam the body on their own and are more specific. IgG antibodies remain in the body for sustained periods of time and are the key to building immunity against a virus. The presence of the different antibodies acts as a timeline of infection: IgM indicates recent infection, followed by both IgG and IgM, and lastly IgG only (E). However, at this time scientists are not sure how long this timeline is or if the presence of specific IgG antibodies will provide a patient with immunity to reinfection.
The process of developing antibodies against a specific virus is known as seroconversion. A patient who has no antibodies against the virus in their blood serum is seronegative, while a patient with antibodies in their serum is seropositive. The COVID-19 serology test determines if a patient has undergone seroconversion.
How The Test Works
The majority of approved serology tests in the U.S. are classified as RDTs, or rapid diagnostic tests. Scientists refer to this test as a lateral flow immunoassay (LFI) and this technology as a lateral flow device (LFD). While other tests exist, these tests are relatively inexpensive and quick to deliver results, making them the most popular.
LFDs utilize a physical principle called capillarity: liquids flow in narrow spaces without needing energy. This principle is common in laboratory research and is the same technology used in pregnancy tests. The COVID-19 serology test uses capillarity to visually represent whether a patient’s blood sample contains antibodies specific to components of the SARS-CoV2 “S” protein. The test represents this through color, although fluorescence and magnetism can also be used in other tests.
The test starts by placing the patient’s blood sample at one end of the test strip (1). Importantly, the test strip contains pads that soak up the blood sample and allow it to flow from one end of the strip to the other via capillary action. At the beginning of the strip, the conjugate pad contains pieces of SARS-CoV2 “S” protein attached to colored probes. If the patient’s blood sample contains SARS-CoV2 antibodies, they will attach to these “S” protein color probes. The sample moves down the strip with the colored “S” protein attached to any SARS-CoV2 antibodies and then meets a row designed to detect IgM antibodies. If the patient sample contains SARS-CoV2 IgM antibodies, these antibodies will be stopped in this region and the color from the attached “S” protein will become visible as a line on the strip (2). Next, the sample comes across a row to detect IgG antibodies, which works in the same manner as IgM. Lastly, a control row detects control probes which moved with the patient sample throughout the test. The control line confirms that the test worked correctly (3). Note, the order of IgM and IgG varies by the manufacturer, but the principle is the same.
After the sample has moved across the entire strip, the results can be read (4). If the test is negative for both IgG and IgM and the control line is colored, the patient was never exposed to SARS-CoV2 (4, 4th from Left). If any of the antibody lines are positive, the presence of the different antibodies acts as a timeline of infection. As explained above: IgM indicates recent infection (4, 1st on Left), followed by both IgG and IgM (4, 2nd from Left), and lastly IgG only (4, 3rd from Left). However, if any of the IgG or IgM strips are positive but the control strip is not, the test is invalid (4, 5th& 6th from Left).
For more information on the serology testing environment and a dive into the science of immune activation and testing accuracy, read the engaging editorial posted in Science Translational Medicine by Derek Lowe, PhD. Additionally, check out this recent New York Times article for the essentials of antibody testing and what results actually mean for re-starting aspects of society.




